Research
The Tabula Rasa lab develops, validates, and implements biomarkers for neuro-oncologic and neuro-genetic diseases. These biomarkers are critical to improving the care for patients with neurological diseases such as glioblastoma, which lack cures or treatments that are effective in the long-term. By developing biomarkers for neurologic diseases, we hope to diagnose these conditions earlier, estimate prognosis more accurately, identify when treatments are and are not working, and improve how clinical trials are run. Neurologists use signs and symptoms and the clinical history reported by the patient or caregiver to infer the disease process, its etiology, disease progression, and its response to treatment. Biomarkers quantify these changes and can help to identify specific disease subtypes, such as those that are more likely to respond to a particular treatment. Our lab uses applied mathematics, computer science, data science, genomics, wet lab biology, and patient registry studies to identify and test biomarkers. We aim to improve patient quantity and quality of life using biomarker-driven neurology.
Gene Signatures and Non-Coding RNA
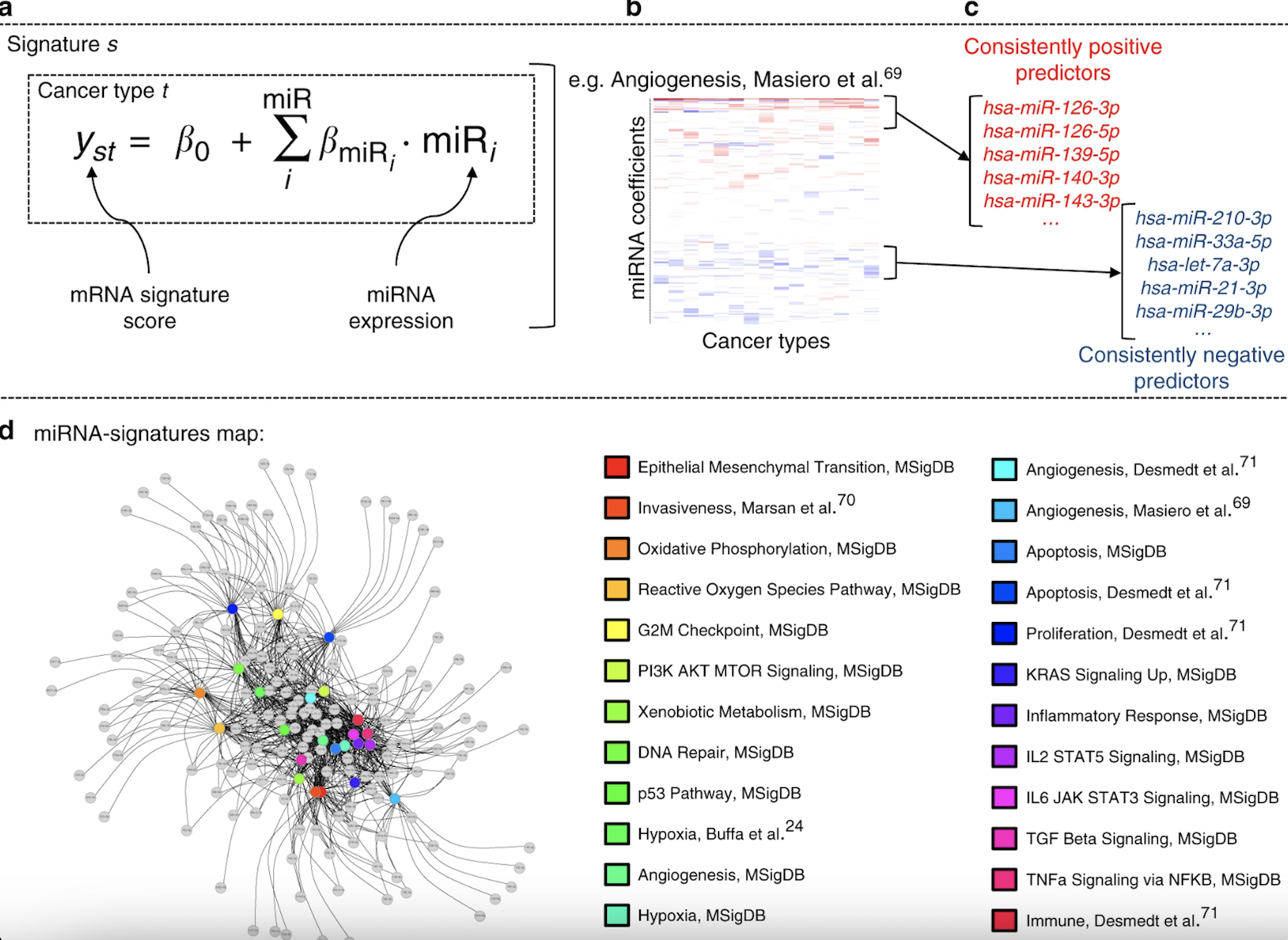
The genomic revolution has provided incredibly detailed mechanistic insights into how individual cells behave in cancers, and how these patterns of activity drive disease. Understanding these large genomic datasets has relied on gene signatures, patterns of gene expression that indicate a phenotype of interest. Gene signatures have been developed for myriad biological phenomena: prognosis, cellular state, and diagnosis. However, most published gene signatures have not shown biological or clinical relevance outside the original datasets from which they were derived.
We seek to identify new gene signatures, and new methods of developing gene signatures, with clinical relevance to bring these powerful tools to the clinic to help physicians more effectively prognosticate and treat disease. Specifically, we are interested in how non-coding RNA expression may function as a biomarker for neurological disorders. miRNA and circular RNA (circRNA) signatures may be detected readily in various biofluids and may help diagnose neurologic diseases earlier than alternative methods. We seek to identify specific miRNA and circRNA signatures that can function as potential diagnostic and therapeutic markers, using cerebrospinal fluid, blood, and tissue assays.
Disease Risk Assessment and Prediction
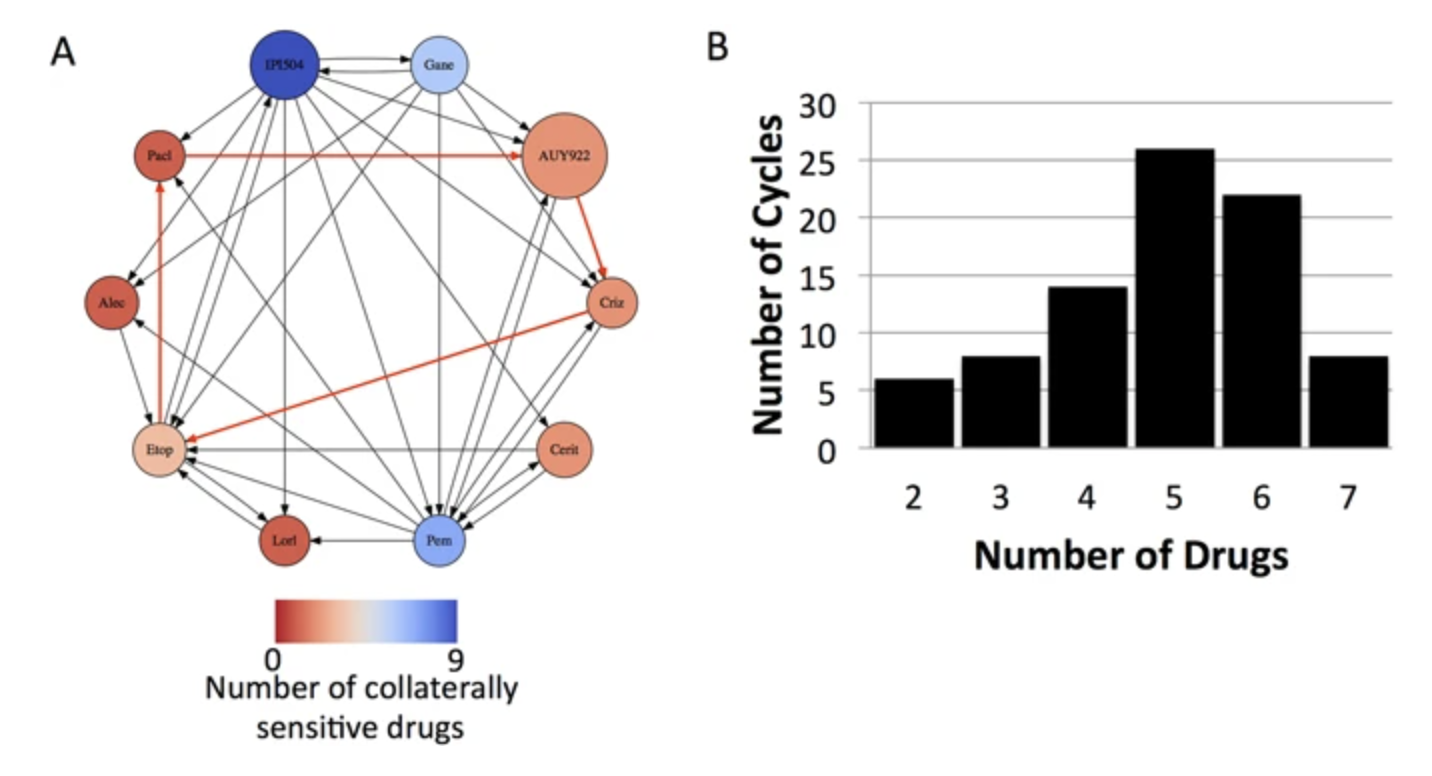
Our lab engages in broad collaborations aimed at developing comprehensive risk models for disease complications and progression. By integrating genomic profiling, imaging, and clinical data, we strive to identify predictors for specific outcomes or disease subtypes. This multifaceted approach allows us to create precise risk models that can significantly enhance patient care and treatment strategies. In addition to clinical data, we also seek to use wearable devices in patient care to better understand how disease impacts function, and whether continuous remote monitoring, in diseases like glioblastoma, can improve outcomes and care.
Clinical and Translational Research
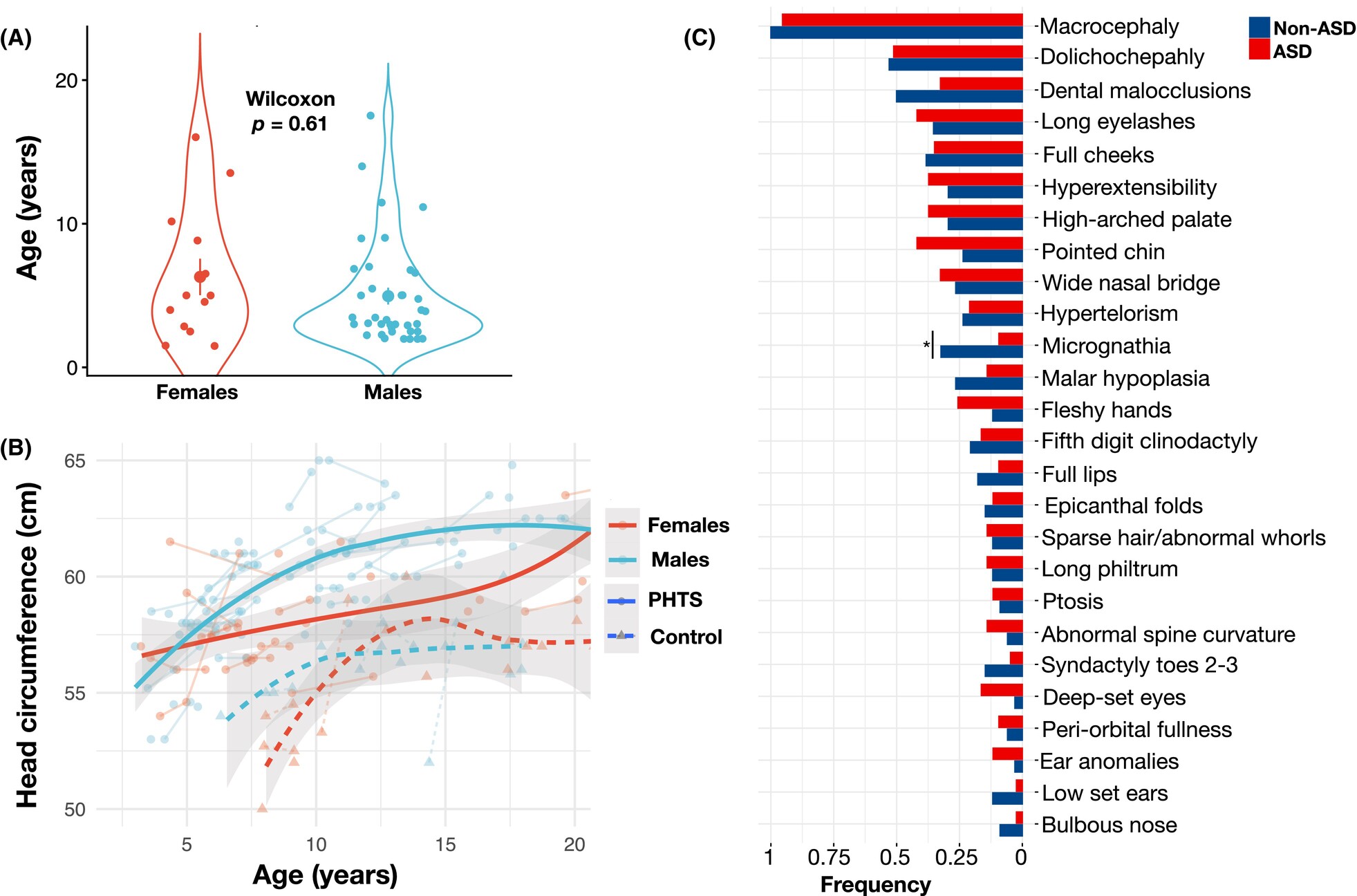
A further focus of our lab is the care for individuals with rare neurologic disorders. Dr. Dhawan has been involved in the care for individuals with PTEN hamartoma tumor syndrome, a rare genetic disorder leading to a predisposition towards tumors and neurodevelopmental challenges. The expanding spectrum of neurologic challenges faced by individuals with PTEN hamartoma tumor syndrome is a strong interest of the lab, and we have helped lead the development of clinical care guidelines for the management of neurologic features of this condition.
